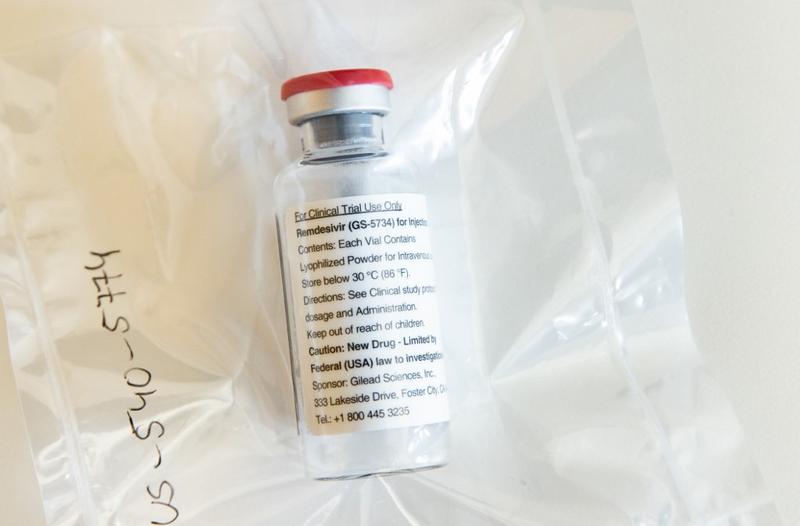
 This file photo taken on April 8, 2020 shows one vial of the drug remdesivir during a press conference about the start of a study in particularly severely ill patients at the University Hospital Eppendorf (UKE) in Hamburg, northern Germany, amidst the COVID-19 pandemic.
(ULRICH PERREY / POOL / AFP)
This file photo taken on April 8, 2020 shows one vial of the drug remdesivir during a press conference about the start of a study in particularly severely ill patients at the University Hospital Eppendorf (UKE) in Hamburg, northern Germany, amidst the COVID-19 pandemic.
(ULRICH PERREY / POOL / AFP)
Japan’s Health Ministry has begun a special approval process for the antiviral drug remdesivir as a potential treatment for COVID-19, NHK reported.
READ MORE: Study: Two-thirds of patients improve after using Remdesivir
The approval process could be completed in around a week, the public broadcaster said without citing anyone. The move by Japan comes just after US regulators cleared the drug for emergency use in COVID-19 patients.
Remdesivir would be the first COVID-19 treatment drug available in Japan if it is cleared for use. With limited supplies, it’s likely to only be available at specific hospitals under the ministry’s jurisdiction during early stages, NHK said.
ALSO READ: Coronavirus: When will the cure be ready?


