 A laboratory technician collects vials of patient swabs during coronavirus detection testing at a laboratory in the Torlak Institute of Virology in Belgrade, Serbia, on March 4, 2020. (OLIVER BUNIC/ BLOOMBERG)
A laboratory technician collects vials of patient swabs during coronavirus detection testing at a laboratory in the Torlak Institute of Virology in Belgrade, Serbia, on March 4, 2020. (OLIVER BUNIC/ BLOOMBERG)
Sinopharm Group Co. surged by the most since April 2015 to lead a rally among Chinese healthcare names, as the trial results for its COVID-19 vaccine candidate rekindled investors’ enthusiasm for the sector.
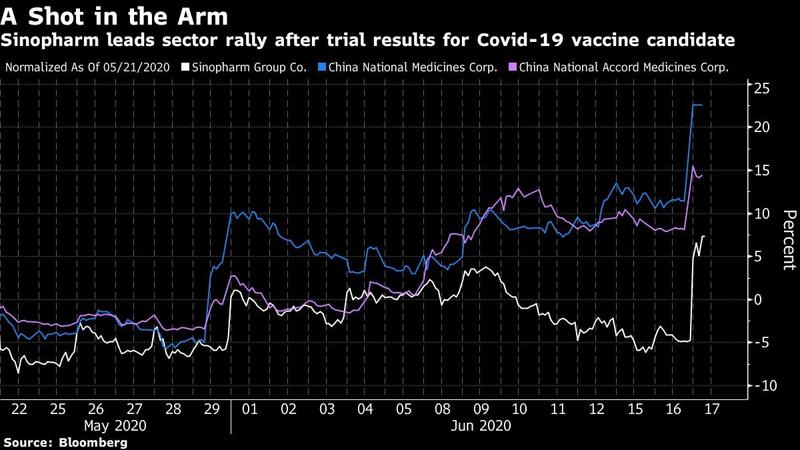
China National Medicines Corp., a unit of Sinopharm, soared by the 10 percent daily limit in Shanghai while another subsidiary China National Accord Medicines Corp. jumped as much as 8.8 percent in Shenzhen
The vaccine candidate, developed by Wuhan Institute of Biological Products Co, has shown no serious adverse reactions during phase I and II clinical trials, according to a Weibo posting Tuesday by China National Biotec Group, a subsidiary of the company’s parent. That sent Sinopharm shares up by as much as 15 percent in Hong Kong before finishing 9.2 percent higher.
China National Medicines Corp, a unit of Sinopharm, jumped the 10 percent limit in Shanghai while another subsidiary, China National Accord Medicines Corp, also rose the daily maximum in Shenzhen. Six of the top 10 gainers in the Asia Pacific region were Chinese pharmaceutical or biotechnology firms, including Sinopharm and the two units. Meanwhile, the health-care segment in the MSCI China Index rose 2.1 percent, hitting a fresh two-year high.
ALSO READ: Sinopharm unit nears vaccine breakthrough
The vaccine involved 1,120 volunteers aged between 18 and 59 in its clinical trials, which started on April 12.
The results revealed a good safety record, with no cases of severe adverse effects found in the clinical trials. The vaccine receivers inoculated with two injections in different procedures and doses have all produced high titers of antibodies.
For those receiving two middle-dose injections at intervals of 14 days and 21 days, the seroconversion rate of neutralizing antibodies reached 97.6 percent. For those receiving two middle-dose injections at an interval of 28 days, the seroconversion rate of neutralizing antibodies reached 100 percent.
The CNBG is actively promoting overseas cooperation in the phase 3 clinical trial of the vaccine and has secured the intention of cooperation of several companies and research institutions from other countries.
The company has also built a production workshop with high-level biosafety, which could help ensure the supply of vaccines for emergency use.
READ MORE: Candidate vaccine has shown promise in early trials
China has promised to share any successful vaccine globally.


