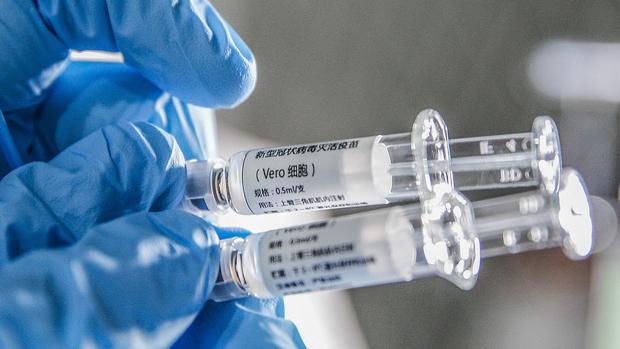
 A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd, in Beijing, capital of China, March 16, 2020. (ZHANG YUWEI / XINHUA)
A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd, in Beijing, capital of China, March 16, 2020. (ZHANG YUWEI / XINHUA)
BEIJING – An inactivated COVID-19 vaccine candidate developed by the Institute of Medical Biology under the Chinese Academy of Medical Sciences has entered phase-2 clinical trials in China, the Science and Technology Daily reported Saturday.
ALSO READ: Official: China to make COVID-19 vaccine global public good
The phase-2 trials, which further evaluate the immunogenicity and safety of the vaccine in humans, are being conducted in the southwestern province of Yunnan.
READ MORE: Vaccine R&D not competition, says Foreign Ministry
So far, five COVID-19 vaccine candidates have been approved for clinical trials in China, accounting for 40 percent of the total vaccines in clinical trials worldwide, according to the Ministry of Science and Technology.



