Traditionally, organizations like Gavi, which helps lower-income countries buy vaccines, can only start purchasing shots once they have approval from the WHO. But the rules have been relaxed in this instance to get talks moving, as the WHO's approval is due in a few weeks.
Two vaccines, made by Denmark's Bavarian Nordic and Japan's KM Biologics, are already approved by regulators around the world, including the United States and Japan, and have been in widespread use for mpox since 2022.
ALSO READ: Thailand confirms mpox case is clade 1b, second outside of Africa
Around 1.2 million people have had Bavarian Nordic's vaccine in the United States alone. The WHO is expected to grant an emergency license to the shots in September.
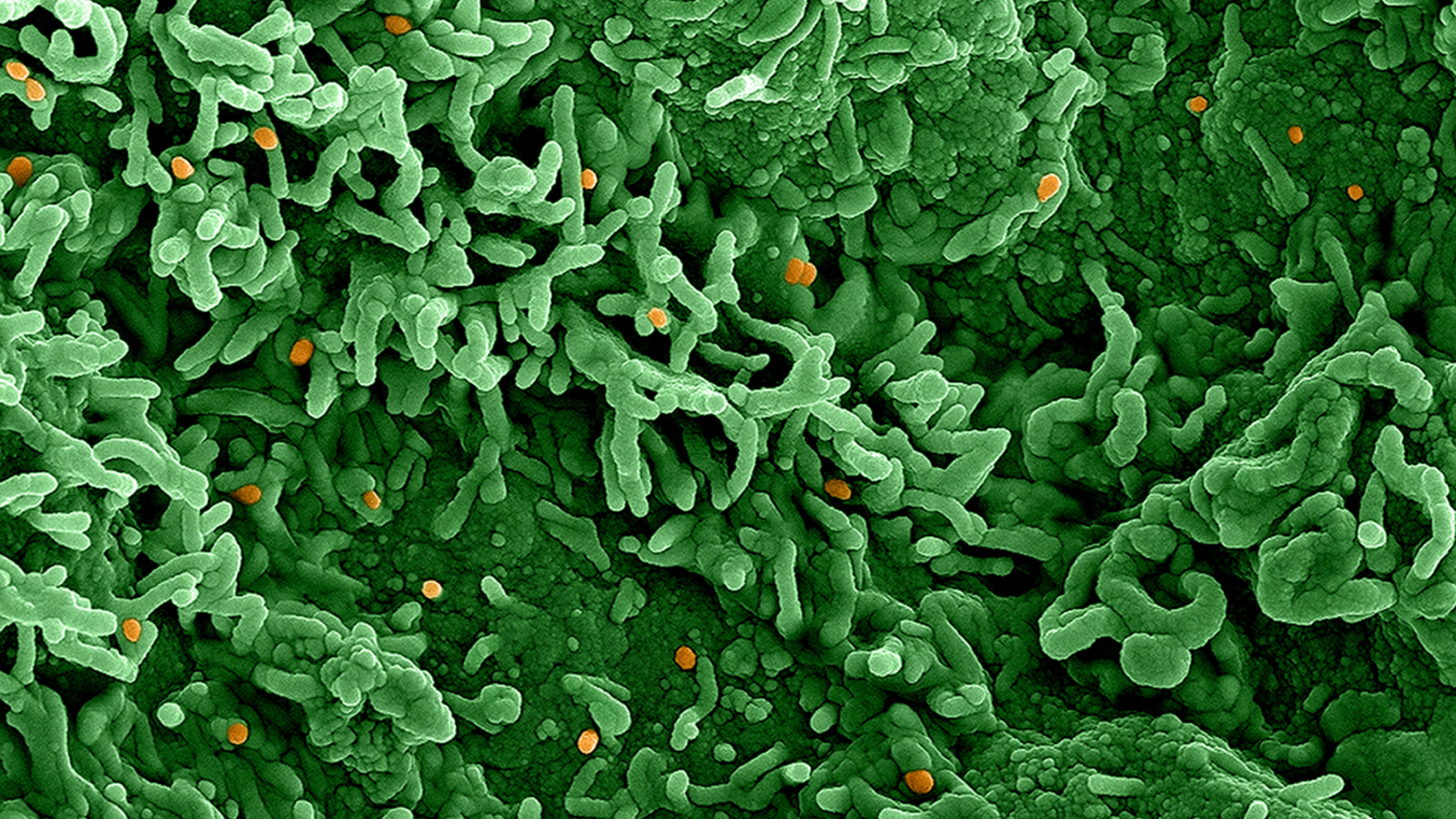
Mpox, a viral infection that spreads through close contact and is usually mild but can kill, was declared a public emergency of international concern by the WHO last week after a new offshoot of the virus spread quickly in Democratic Republic of Congo and beyond.
Earlier this month, the WHO asked vaccine manufacturers to submit information so it could accelerate its approval process, and grant an emergency license by mid-September.
However this week, one of the vaccine manufacturers, Bavarian Nordic, said it needed orders immediately from organizations like Gavi and the WHO to make more shots this year, raising fears that lower-income countries could miss out or be forced to rely once again on precarious donations from high-income countries, as happened during the COVID-19 pandemic.
READ MORE: WHO confirms first case of new mpox strain outside Africa
Some donated mpox vaccines are due to arrive in Africa next week, the Africa Centres for Disease Control and Prevention has said.


